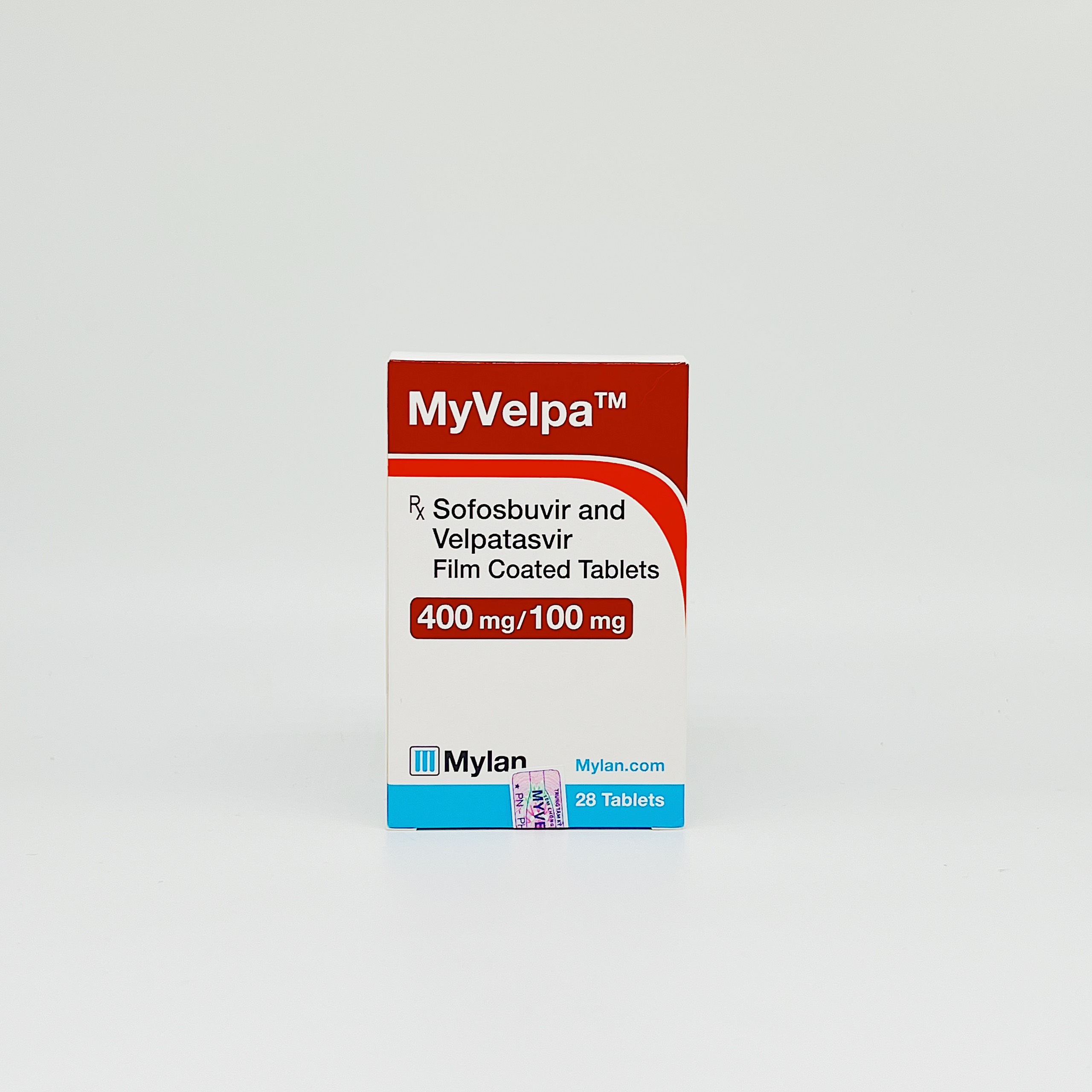
Myvelpa
Description
Ingredients:
Sofosbuvir, Velpatasvir
Excipients:
Drug Excipients: Copovidone, Lactose Monohydrate, Microcrystalline Sodium (Ceolus UF 702), Microcrystalline Cellulose (Avicel PH 112), Croscarmellose Sodium, Colloidal Silicon Dioxide, Magnesium Stearate Coating Systems Excipients (Green TC-510031): Polyvinyl Alcohol, Polyethylene Glycol, Titanium Dioxide, Talc, Indigo carmine Aluminum lake, Iron Oxide Yellow
Assign:
Velpatasvir/sofosbuvir tablets are indicated for the treatment of chronic hepatitis C virus infection in adults.
Expiry date:
24 months
Packing:
Bottle of 28 tablets
Preservation:
Drugs are to be stored in a cool and dry place, below 30°C. Store in original packaging. Keep out of reach of children. Read the user manual carefully before use.
Brand Origin:
India
Products Details:
Amount use
Take 1 tablet (400mg sofosbuvir/100mg velpatasvir) daily, with or without food.
Contraindications:
Patients with hypersensitivity to the components of the drug and pregnant women.
Careful and informed
- Do not use velpatasvir/sofosbuvir tablets concurrently with other medicinal products containing sofosbuvir. - Bradycardia and severe heart block. - The patient has failed a treatment regimen containing NS5A. - Patients with kidney failure. - The patient is taking moderate P-gp and CYP inducers. - Use in combination with HIV treatment regimens. - Patients are coinfected with HCV and HBV (hepatitis B). - Patients with cirrhosis of the liver CPT index C. - Liver transplant patient.
See details