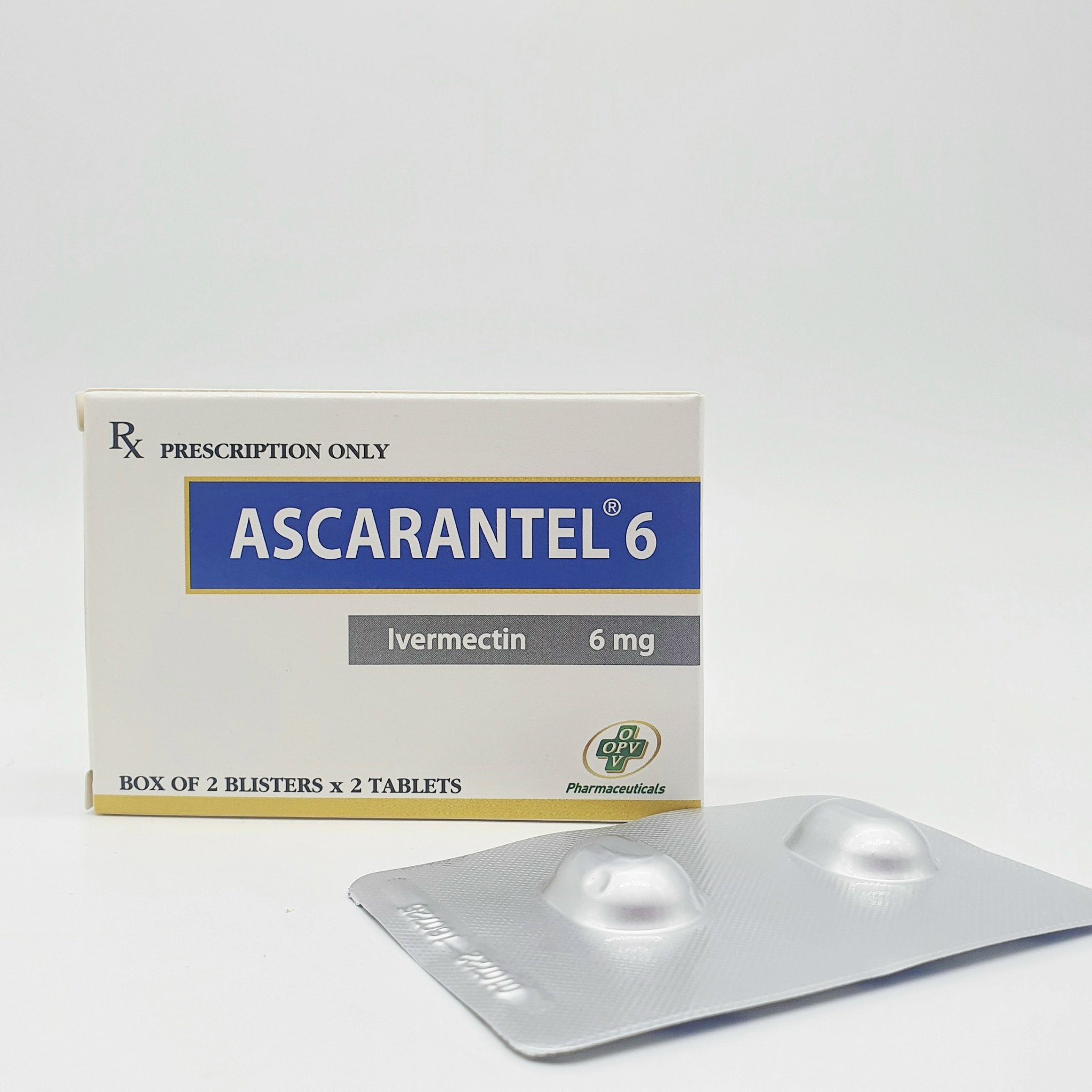
Tenifor
Description
Ingredients:
Tenofovir Disoproxil Fumarat
Excipients:
Microcrystalline cellulose, dibasic calcium phosphate, lactose, povidone K30, purified talc, magnesium stearate, aerosil, sodium glycolate starch, corn starch, hypromellose, titanium dioxide, brilliant blue.
Assign:
- Treatment of HIV infection: According to the guidelines of the Ministry of Health, TENIFO is indicated in combination with other antiretroviral drugs to treat HIV-1 infection in adults and children over 10 years old, pregnant and breastfeeding women. - Hepatitis B: TENIFO is indicated for the treatment of hepatitis B in adults with compensated liver function, with evidence of viral replication activity, persistently elevated alanine aminotrasferase (ALT) levels, and tissue evidence. of active inflammation and/or fibrosis.
Expiry date:
36 months
Packing:
Box of 1 blister of 10 tablets
Preservation:
Store in a cool and dry place below 30°C, protected from light.
Brand Origin:
India
Products Details:
Amount use
Adults and children over 35 kg: The recommended dose is 300 mg (1 tablet) once a day with meals. Children: Target dose for children over 10 years of age (<35 kg): 8 mg/kg or 200 mg/m2 (maximum 300 mg). Elderly: There are no data on dosing in elderly patients over 65 years of age. Patients with renal impairment: Dosage intervals should be adjusted for patients with creatinine clearance < 50 ml/min as directed by the physician.
Contraindications:
Patients who are sensitive to the components of the drug.
Careful and informed
It must not be used together with any other medicine containing tenofovir disoproxil fumarate. CKD. Liver failure. Lactic acidosis. Immune response syndrome.
See details